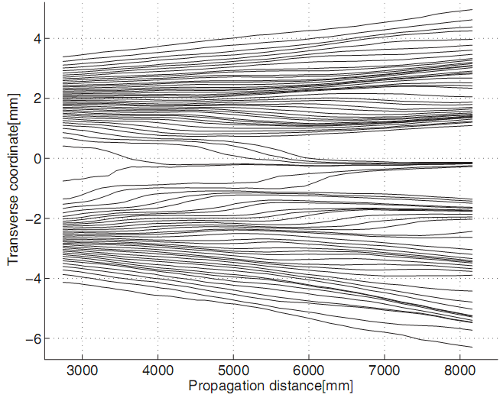
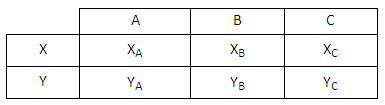
Why Quantum Statistics?
Why Quantum Statistics?Consider the following scenario. An initial measurement indicates that two indistinguishable particles...
 To Be Is To Be Measured (III: Contextuality)
To Be Is To Be Measured (III: Contextuality)In their seminal paper of 1935, Einstein, Podolsky, and Rosen (EPR) put forward the following criterion...
 The 2-Slit Experiment Revisited (2: Interpretation And Why It's So Hard...)
The 2-Slit Experiment Revisited (2: Interpretation And Why It's So Hard...)Continued from the last post.Discounting the Bohmian song and dance, we are led to conclude that...
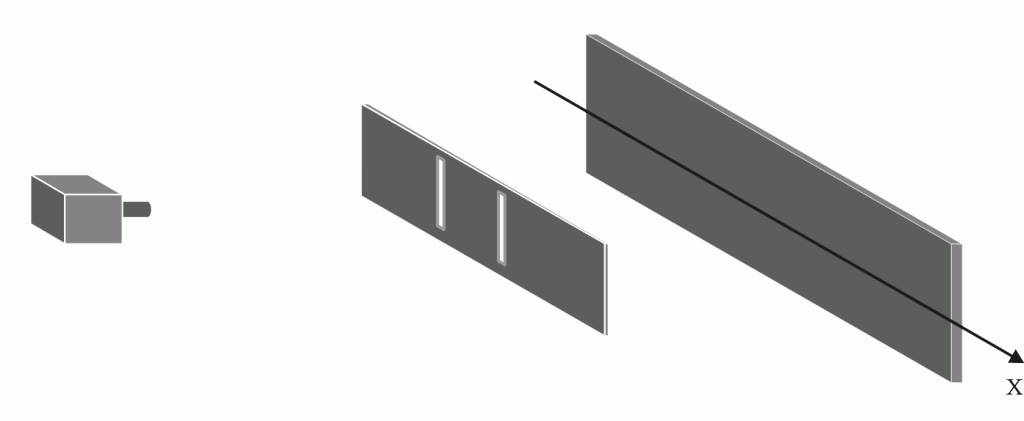 The 2-Slit Experiment Revisited (1: Calculation)
The 2-Slit Experiment Revisited (1: Calculation)According to Feynman, the 2-slit experiment with electrons "has in it the heart of quantum mechanics"...